Với những anh em yêu thích hình thức cá cược online thì Thabet là một địa chỉ quen thuộc. Còn riêng với những anh em mới gia nhập giới lô đề thì nên tìm hiểu kỹ về nhà cái này trước khi tham gia.
Thabet là gì?
Nếu thường xuyên lướt web đọc tin tức hẳn bạn sẽ thấy vài chỗ nhắc đến trang Thabet. Tuy nhiên đối với nhiều người tên miền này còn rất xa lạ, chỉ khi đầu tư thời gian, công sức tìm hiểu bạn mới nhận ra nó lạ mà quen.
Trên thực tế Thabet là một trong những tên miền chuyển hướng được quản lý bởi nhà cái Thabet nên có tất cả các dịch vụ y như web mẹ. Sở dĩ Thabet lập ra các trang web chuyển hướng như Thabet, Ku, Ku999… để giảm tình trạng quá tải ở trang chủ. Chính vì vậy bạn không nên tin vào lời đồn là trang web này lừa đảo như nhiều kẻ thêu dệt.
Tại trang web chuyển hướng này bạn có thể trải nghiệm những trò chơi cá cược một cách mượt mà nhất. Đồng thời bạn vẫn được hưởng trọn các chương trình khuyến mãi hấp dẫn như trang mẹ Thabet. Nếu ngay từ đầu bạn đã tạo tài khoản trên trang Thabet thì những lần sau bạn nên tiếp tục chơi game trên trang.
Thabet có uy tín không?
Khi tham gia cá cược bạn sẽ phải bỏ ra một khoản tiền vốn khá lớn, vậy nên mọi người thường cẩn thận trong việc lựa chọn địa chỉ chơi game, tránh gặp lừa đảo. Bên cạnh tiền bạc, nếu gặp web ảo thì bạn còn gặp nỗi lo về thông tin cá nhân bị rò rỉ.
Thị trường cá cược online đang phát triển bùng nổ nên có rất nhiều nhà cái mới được thành lập. Tốt nhất bạn nên tìm đến các nhà cái có uy tín lâu năm, được người chơi đánh giá cao như Thabet, và tất nhiên Tha bet – web con cũng là sự lựa chọn đáng tin. Với những anh em mê mệt cá cược thì Thabet – Thabet là những cái tên hết sức quen thuộc.
Thabet vốn là nhà cái có trụ sở tại Philippines, được cấp phép và quản lý chặt chẽ nên luôn đảm bảo quyền lợi cho người chơi. Hoạt động cờ bạc đã được hợp thức hóa tại nước sở lại nên bạn có thể hoàn toàn yên tâm khi chơi game tại Thabet.
Tuy Thabet có nhiều tran con khác nhau nhưng tựu chung chỉ có một địa chỉ quản lý, chung các chương trình khuyến mãi hấp dẫn nên khi tham gia bạn không lo thiệt thòi. Những thông tin như Thabet lừa đảo hoàn toàn là tin đồn nhảm do đối thủ cạnh tranh lan truyền, không đáng tin cậy. Nhìn chung đây là địa chỉ tin cậy, rất đáng để chọn mặt gửi vàng khi tham gia cá cược.
Các trò chơi hấp dẫn tại Thabet
Thabet đã trở thành địa chỉ tin cậy dành cho rất nhiều anh em ham thích cá cược. Một trong những lý do giúp nhà cái này có sức hút lớn như vậy là do khi trò chơi hấp dẫn, đa dạng. Có thể kể đến những trò chơi hấp dẫn như:
Lô đề, xổ số online
Lô đề online là trò chơi được yêu thích bậc nhất tại Việt Nam với lịch sử lâu đời. Thế nhưng hình thức chơi lô đề truyền thống giờ đã lỗi thời vì tốn nhiều thời gian, công sức cho việc đi lại từ nhà ra đại lý. Hơn thế nữa thì tỷ lệ trả thưởng tại các đại lý truyền thống cũng không quá hấp dẫn.
Trong thời gian gần đây lô đề online đã trở thành sự lựa chọn của đa phần anh em chơi lô đề. Bạn có thể chơi lô đề chỉ với một chiếc máy tính, điện thoại thông minh có kết nối mạng. Thabet chính là sự lựa chọn tuyệt vời khi có đầy đủ các hình thức cá cược lô đề cho bạn lựa chọn: lô 2 càng, 3 càng, lô thường, lô xiên… Tỷ lệ trả thưởng tại Thabet cực kỳ hấp dẫn, ví dụ như đề 2 càng là 1 ăn 99.
Để giúp bạn có thể dễ dàng thắng cuộc trong trò chơi lô đề, Thabet còn cung cấp các tiện ích như soi cầu, nghiên cứu lô đề… đến từ chuyên gia. Bạn có thể tham khảo các ý kiến này để lựa chọn được bộ số tốt nhất chơi lô đề.
Cá độ thể thao online
Tương tự như website mẹ Thabet thì Thabet cũng cung cấp đầy đủ các loại hình cá cược dành cho mọi môn thể thao: bóng đá, bóng chuyền, bóng rổ… Hơn thế nữa đối với từng bộ môn nhà cái luôn tổ chức cá cược cho các giải đấu lớn và quan trọng.
Riêng với bóng đá – môn thể thao vua thì càng không có gì để chê, tùy theo chiến thật của mình mà bạn có thể đặt cược cho kèo Châu Á, kèo Châu Âu…

Bạn có thể tham gia cá độ thể thao
Slot game với phần thưởng hấp dẫn
Slot game tại Thabet cũng nhận được rất nhiều lời khen ngợi với hình ảnh độc đáo, âm thanh sống động. Bạn có thể tham gia trò chơi này để kiếm thêm một khoản tiền lớn đồng thời tha hồ giải trí. Bên cạnh nổ hũ thì bắn cá 3D cũng là tựa game hấp dẫn, rất đáng tham gia.
Cá cược thể thao điện tử esports
Bên cạnh cá cược thể thao thì cá cược điện tử cũng đang dần chiếm được cảm tình của người chơi, đặc biệt là những người trẻ tuổi. Bạn sẽ có cơ hội thu bội điện môi khi mùa giải thể thao điện tử bắt đầu khởi tranh.
Thể thao điện tử ngày càng phát triển mạnh mẽ, kéo theo đó là rất nhiều giải đấu được tổ chức thường xuyên. Những người mê điện tử thứ thiệt chắc chắn sẽ không muốn bỏ qua giải đấu đẳng cấp này.
Casino online
Không phải ai cũng có điều kiện tham gia cá cược tại casino trực tiếp do không có thời gian đi lại, không có đủ điều kiện tài chính để vào sòng. Thay vào đó bạn có thể tham gia vào các trò chơi tại casino online của Thabet với hệ thống trò chơi đa dạng. Có thể kể đến như: Rồng hổ, tài xỉu… Đây chính là thiên đường dành cho những người mê cờ bạc.
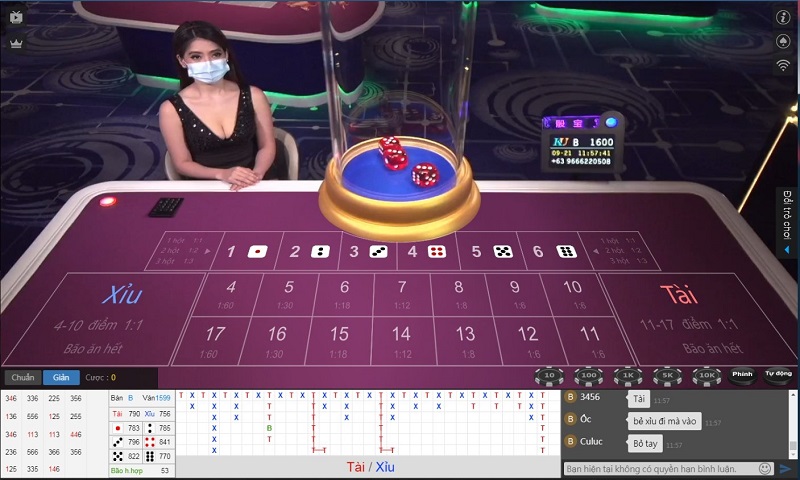
Tham gia casino online hấp dẫn
Tại sao bạn nên tham gia chơi game tại Thabet
Hiện nay Thabet đã thu hút một lượng lớn người chơi cá cược tại Việt Nam tham gia. Ngoài kho trò chơi phong phú và hấp dẫn kể trên thì nhà cái này còn có rất nhiều ưu điểm nổi bật, điển hình như:
Live stream hot girl hấp dẫn
Một điểm đặc biệt của nhà cái Thabet là anh em sẽ được tham gia chơi và trò chuyện trực tiếp với các hotgirl nóng bỏng. Điều này sẽ giúp bản cảm thấy chân thực và vui vẻ trong suốt quá trình chơi game.
Tỷ lệ trả thưởng hấp dẫn, nhiều khuyến mại cho người chơi
Nếu muốn tối ưu hóa nguồn lợi nhuận thu được thì bạn cần chọn nhà cái có tỷ lệ trả thưởng cao và chắc chắn Thabet là sự lựa chọn tối ưu nhất. Hiện nay Thabet là một trong những nhà cái có tỷ lệ trả thưởng cao nhất thị trường, ví dụ như đánh đề là 1 ăn 99. Hơn nữa khi thành lập tài khoản thì bạn sẽ nhận được vô vàn khuyến mãi hấp dẫn.
Ví dụ như nhà cái sẽ khuyến mại 20% cho lần đầu nạp tiền, vào các dịp đặc biệt nhà cái còn tặng cho người chơi rất nhiều giftcode giá trị.
Nhân viên hỗ trợ nhiệt tình 24/7
Không phải ai cũng quen với việc cá cược online, nhất là những người cưa rành công nghệ. Hiện nay Thabet sở hữu đội ngũ nhân viên chăm sóc khách hàng chuyên nghiệp nhiệt tình nên bạn sẽ được phục vụ tốt nhất. Nếu bạn gặp phải bất kỳ sự cố nào, hãy liên hệ với nhân viên hỗ trợ ngay để được xử lý nhanh nhất.
Nạp rút tiền nhanh chóng
Nhà cái Thabet liên kết hầu hết với tất cả các ngân hàng lớn tại Việt Nam nên bạn có thể nạp rút tiền dễ dàng. Tiền sẽ được chuyển vào tài khoản chơi game vài giây nên sẽ không làm gián đoạn quá trình cá cược của bạn. Hơn nữa ngay khi có kết quả nhà cái sẽ tiến hành trả thưởng ngay, bạn có thể rút về tiêu thoải mái.

Thabet cho phép bạn nạp rút tiền nhanh chóng
Trải nghiệm mượt mà, dễ dàng thao tác
Một điểm thú vị tại nhà cái là quá trình thực hiện các thao tác cá cược vô cùng mượt mà, đảm bảo không bị gián đoạn. Do đó bạn có thể thoải mái chơi game, không sợ lỡ mất ván cược đắt giá nào.
Hướng dẫn cách đăng ký, đăng nhập tài khoản tại Thabet
Quá trình đăng ký, đăng nhập tài khoản tại Thabet cực kỳ đơn giản và dễ dàng, cụ thể quy trình thực hiện như sau:
Quy trình đăng ký tài khoản
- Bước 1: Truy cập vào link nhà cái, ấn vào nút đăng ký.
- Bước 2: Nhập đầy đủ các loại thông tin mà nhà cái yêu cầu, đợi mã xác nhận được gửi vào máy điện thoại mà nhập lại mã xác nhập.
- Bước 3: Bổ sung tài khoản ngân hàng để dễ dàng nạp rút tiền, nhập mật khẩu rút tiền.
Quy trình đăng nhập tài khoản
- Bước 1: Truy cập vào link nhà cái, ấn vào nút đăng nhập.
- Bước 2: Nhập tên tài khoản và mật khẩu.
- Bước 3: Ấn nút đăng nhập.
Nhìn chung nhà cái luôn có các bài viết cụ thể để hướng dẫn sử dụng tài khoản cá cược, bạn nên đọc để thao tác cho chính xác.
Thabet là địa chỉ tin cậy dành cho các anh em yêu thích cá cược tại Việt Nam với giao diện dễ thao tác và tỷ lệ trả thưởng hấp dẫn. Trang web này cực kỳ uy tín nên bạn không phải lo lắng bất kỳ vấn đề gì cả, cứ yên tâm tham gia cá cược.